Abstract
BACKGROUND: Despite advances in the understanding of disease pathogenesis, improved treatment strategies, and evidence of better survival with treatment, a large proportion of older patients (pts) with acute myeloid leukemia (AML) remains untreated and has poor outcomes. The aim of this study is to describe current treatment patterns in pts with AML aged <60, 60-64, and ≥65 years (yrs), and assess healthcare resource utilization (HRU) in pts with AML aged <65 and ≥65 yrs in a real-world claims database.
METHODS: AML pts were retrospectively identified in the MarketScan® Research Database (Truven Health) including both Commercial and Medicare Supplemental claims databases between 01/2011 to 07/2016. Pts with ≥2 AML diagnoses, ≥1 bone marrow procedure (the first AML diagnosis following the procedure was defined as the index date), and ≥12 months of continuous eligibility (baseline period) prior to the index date were included. Exclusion criteria were ≥1 diagnosis for AML relapse or remission on or before the bone marrow procedure, ≥2 diagnoses for another blood cancer, and ≥1 allogeneic hematopoietic stem cell transplantation before the bone marrow diagnosis procedure. Furthermore, pts aged ≥65 yrs at the index date were excluded if they had ≥1 claim for intensive chemotherapy at any time. The observation period spanned from index date up to the first of health plan disenrollment or the end of data availability. To accommodate different age cut-offs in accordance with National Comprehensive Cancer Network treatment guidelines, top treatments (excluding hormonal therapy agents) received post-AML diagnosis were reported in pts aged <60, 60-64, and ≥65 yrs. Listings that lacked codes for specific chemotherapy agents, but offered adequate International Classification of Diseases codes were included and reported as "unknown antineoplastic agents". HRU, including days of hospital stay, days with outpatient services, days with emergency room visits, days with hospice care, and days with durable medical equipment use, was evaluated in pts <65 and ≥65 yrs old. To account for varying length of follow-up among pts, HRU was reported per patient per month.
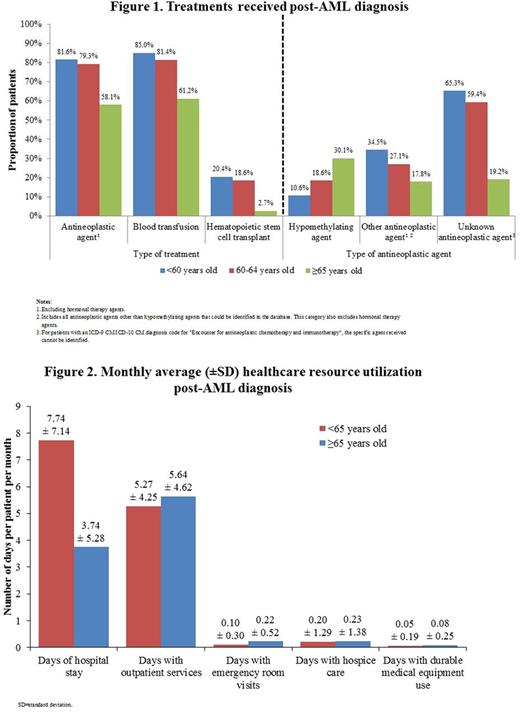
RESULTS: After applying the selection criteria, 2,954 AML pts were identified: 1,492 pts ≥65 yrs old (mean [SD] age: 76.8 [7.0]; 36.6% were ≥80 yrs old; mean [SD] follow-up: 212 [255] days) and 1,462 pts <65 yrs old (mean [SD] age: 51.8 [11.5]; mean [SD] follow-up: 350 [350] days), including 468 (32.0%) aged 60-64 yrs and 994 aged <60 yrs. Pts <60 yrs old received the most therapeutic interventions (i.e., ≥1 antineoplastic agent, ≥1 blood transfusion, or ≥1 hematopoietic stem cell transplant), followed by pts 60-64 and pts ≥65 yrs old (Fig. 1). Type of antineoplastic agents (excluding hormonal therapy agents) received varied by age group with "other antineoplastic agent" (other than HMAs and excluding hormonal therapy agents) and "unknown antineoplastic agent" more commonly used in younger pts. Hypomethylating agents (HMAs) were more commonly used in older pts (Fig. 1). In pts aged <60, 60-64, and ≥65 yrs treated with ≥1 HMA, azacitidine was given in 56.2%, 59.8%, and 55.7% of pts and decitabine was given in 50.5%, 47.1%, and 51.2%, respectively. In pts <60 yrs who received "other antineoplastic agents", tretinoin (31.2%), cytarabine (30.6%), and arsenic trioxide (22.4%) were most commonly used. Pts 60-64 yrs old received cytarabine (30.7%), hydroxyurea (20.5%), and tretinoin (17.3%) as most common agents. Pts ≥65 yrs old received most commonly hydroxyurea (53.4%) and cytarabine (14.3%). Post-AML diagnosis, pts ≥65 yrs old had half the monthly all-cause average number of inpatient days compared to pts <65 yrs old (mean [SD]: 3.74 [5.28] vs. 7.74 [7.14]). Differences in the number of days with outpatient services (mean [SD]: 5.64 [4.62] vs. 5.27 [4.25]) or emergency room visits (mean [SD]: 0.22 [0.52] vs. 0.10 [0.30]; Fig. 2) were smaller.
CONCLUSION: This study revealed that >40% of older AML pts (≥65 yrs) did not receive any antineoplastic therapy. This group also had fewer inpatient days compared to younger AML pts (<65 yrs old). These results show that despite medical advances, a large proportion of older AML pts remains either untreated or undertreated, revealing important unmet needs and suggesting that AML treatment has to face a much-needed expansion to improve disease management in this population.
Lafeuille: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd: Research Funding; Janssen Scientific Affairs, LLC: Research Funding. Sundaram: Johnson & Johnson, LLC: Equity Ownership; Janssen: Employment. Hoehn: Janssen: Employment; Johnson & Johnson, LLC: Equity Ownership. Emond: Janssen Scientific Affairs, LLC: Research Funding. Romdhani: Janssen Scientific Affairs, LLC: Research Funding. Lefebvre: Janssen Scientific Affairs, LLC: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal